
A BONE-ANCHORED PERCUTANEOUS CONNECTOR SYSTEM FOR USE IN COCHLEAR IMPLANTATION

The titanium pedestal is based on existing design features in the EPI Bioglass® implant, developed by UCL, and the Brånemark System®, employed by Nobel Biocare AB, and houses the intracochlear electrode array. The connector system has been designed to provide many safety features whilst offering high speed signal transmission and durability.
Histological data from previous animal studies has shown very good results, both of the osseointegration of the pedestal and the soft tissue response to penetration. The system components are presently undergoing mechanical and electrical testing.
Introduction
A percutaneous connection offers a transparent electrical link
for neural stimulation. In the field of cochlear implants, the
current transcutaneous devices use up to approximately 40% of
their power requirements in the radio frequency link. This link
also places restrictions on the capabilities of the buried electronics
e.g. on pulse rate and bandwidth. A magnet is employed to locate
the link, making the device MRI incompatible without surgery.
There is also the need to check the correct operation of the
device electrodes, particularly in children.
However, the obvious concern in the use of a percutaneous system is the permanent break in the skin, offering a possible pathway for ingress of infection and leak of body fluids. Nobel Biocare currently market percutaneous titanium abutments, employing the Brånemark System®, primarily for use in dentistry and maxillofacial applications but also as a conduction route for a Bone Anchored Hearing Aid® (BAHA®). The percutaneous titanium implants have very good clinical results for the reaction of the surrounding soft tissue and osseointegration (for example Holgers et al., 1994 and Tjellström and Granström, 1995).
Implant Design
The percutaneous pedestal, as well as being accepted by the body,
must also offer sufficient resistance to impact trauma and environmental
degradation. Titanium has for many years been used as an implant
material and has shown excellent biocompatibility and corrosion
resistance.
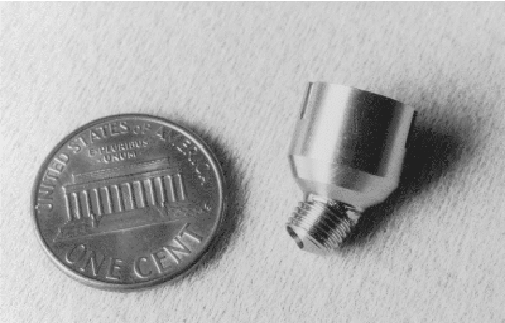
Figure 1 shows the titanium pedestal (Carlsson et al., 1995) against a one cent coin for size comparison. The surgical procedure for cochlear implantation is consistent with current transcutaneous devices except for the fixation of the pedestal. The stem of the pedestal is angled and is placed towards the cochlea, increasing the bending radius of the electrode array. The placement of the stem is in the thickened bone above the sino-dural angle, leaving a bridge of bone between it and the cortical mastoidectomy. A tunnel is then drilled to connect them for the passage of the electrode array. The electrode array is extremely delicate and could suffer damage if the implant was screwed into the bone. To overcome this problem the stem is tapered and is tapped into the tapered hole to provide initial fixation. This angle was found empirically on work on human temporal bones and has been used successfully in the EPI Bioglass® percutaneous connector. The stem has circular grooves, of the Brånemark thread profile, down its length. This allows the pedestal to become fully osseointegrated when bone migration arises with new bone growth. The bone level is at the top of the stem and the soft tissue surrounding the implant is thinned and seats at the angled section. Locating grooves for the external connector can be seen on the outer diameter.
The pedestal requires a very small break in the skin which will be thinned to prevent local movement and minimise the incidence of infection at the site. The low profile of the implant, even when mated with the external connector, should limit the likelihood of trauma damage.
Surgical tooling has been designed for the implant procedure and tested on human temporal bones.
The internal connector plate and the locking ring can be seen in figure 2 (below). The internal connector plate is designed to offer a flat surface to the external connector. Platinum-iridium pins are glassed into an alumina plate and ground flat on the top surface and offer connection pins for the electrode array on the rear. The plate is held by a titanium-alloy locking ring, which also incorporates a profiled groove to accept the spring latching mechanism of the external connector.


Figure 3 shows the external connector, which currently offers 11 active channels. (It is anticipated that with minor design changes the number of channels available will be greatly increased). The connector is based on a commercially proven compression style of interconnection system, offering high speed signal capability. It features sprung plunger pins, a canted-coil latching spring and an earthing mechanism (the displaced 'pin') to discharge any static electricity through the pedestal body and not the electrode array. The connector has appropriate tongues to match the grooves on the pedestal body to offer positive orientation.
The multichannel electrode array is based on proven cochlear implant technology. Two contacts lead to an indifferent electrode and nine contacts lead to the intracochlear array.
Histology Results
The reaction of the soft tissue and bone to the implant has been
assessed in animal studies. To assess the soft tissue response
a full size connector, complete with electrode array, was implanted
into the forehead of adult Yucatan mini-pigs. New Zealand White
rabbits were used in the study for osseointegration, using only
the stem portion of the pedestal implanted subcutaneously in the
tibia.
The histological results were very favourable (Downing et al., 1997), the general structure of the skin indicating a stable condition and excellent bone migration into the grooves of the stem and normal bone structure.
Mechanical and Electrical Testing
The pedestal and connector assemblies are currently undergoing
mechanical and electrical testing.
The electrical leakage current from the implant system to the environment will be monitored during a long term soak test to estimate a mean time to failure. The wear characteristics of the system will be investigated through automated mating of the pedestal assembly and the external connector. The contact resistance and the latching/unlatching force will be monitored during manual mating. The mating tests will be performed under non-sterile conditions using artificial sweat.
Metallographic investigation will follow the completion of all testing procedures.
Clinical Trials
Our current research focuses are to employ the implant with a
multichannel intracochlear electrode array as part of a cochlear
implant system using digital signal processing techniques (Faulkner
et al, 1997), and to study tinnitus suppression using an
extracochlear electrode array to stimulate the cochlear nerve.
The implant system can be adapted for use in other neural prosthesis
applications by tailoring of the electrode array design.
Acknowledgement
The work at UCL is generously supported by grants from The Clothworkers'
Foundation and Defeating Deafness (The Hearing Research Trust).
References
Carlsson, L., Johansson, U., Downing, M., Spraggs, P., Walliker,
J. (1995) Hållarorgan för implantation i benvävnad
(A holder element for implantation in bone tissue). Swedish
patent application no. 95 03555-6, case 4055 SE.
Downing, M., Johansson, U., Carlsson, L., Walliker, J.R., Spraggs, P.D.R., Dodson, H., Hochmair-Desoyer, I.J., Albrektsson, T. (1997) A Bone-Anchored Percutaneous Connector System for Neural Prosthetic Applications. Ear, Nose & Throat Journal vol. 76: 752-755.
Faulkner, A., Walliker, J.R., Rosen, S., Lang, H., Daley, J. (1997) Speech perception using the UCLID CIS cochlear implant speech processor. Speech, Hearing and Language: work in progress vol. 10: 17-26
Holgers, K.-M., Thomsen, P., Tjellström, A., Ericson, L.E., Bjursten, L.-M. (1994) Morphological Evaluation of Clinical Long-Term Percutaneous Titanium Implants. The International Journal of Oral & Maxillofacial Implants vol. 9: 689-697.
Tjellström, A., Granström, G. (1995) One-stage procedure to establish osseointegration: a zero to five years follow-up report. The Journal of Laryngology and Otology vol. 109: 593-598.
© Mark Downing, Ulf Johansson, Lennart Carlsson and John R. Walliker
 for comments
for comments